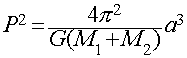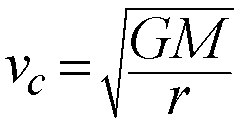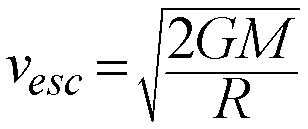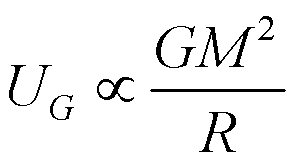|
Astronomy 162:
Introduction to Stars, Galaxies, & the Universe
Prof. Richard Pogge, MTWThF 9:30
|
Matter & Gravity
Readings: Ch 5, section 5-7, Ch 4, sections 4-7 & 4-8
- Structure of Atoms
- Nuclei of protons & neutrons orbited by electrons
- Elements, Isotopes, & Radioactivity
- Four Fundamental Forces
- Strong & Weak Nuclear Forces
- Electromagnetic & Gravitational Forces
- Nature of Gravity
- Inverse Square Law
- Orbits & Circular Speed
- Escape Speed & Gravitational Binding Energy
Matter
Ordinary matter encompasses a wide range of phenomena:
- Fundamental particles (quarks & leptons)
- Subatomic particles (protons & neutrons) composed of fundamental particles
- Single atoms composed of protons, neutrons, & electrons (Hydrogen,
Helium, Gold, etc.)
- Simple molecules composed of 2 or more atoms bound together
- Macromolecules (DNA, complex polymers)
- Macroscopic objects (rocks, people, planets ...)
Most ordinary matter in the universe is in the form of atoms.
Atoms
Atoms consist of two basic parts:
Nucleus of heavy subatomic particles:
- proton: positively charged
- neutron: uncharged (neutral)
Cloud of Electrons orbiting the Nucleus:
- negatively charged particles (leptons)
- masses are 1/1836th the mass of a proton
Atoms are mostly empty space:
- Only 1 part in 1015 of the volume is occupied.
- Volume is threaded by electromagnetic fields.
A simple, yet familiar cartoon:
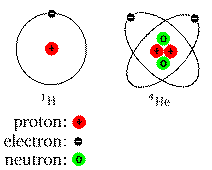
Real atoms don't look like this, but it can help you visualize the
locations and numbers of the different components (nucleus and
electrons).
Chemical Elements
We distinguish atoms into Elements by the total number of protons
in their nucleus.
Atomic Number:
- 1 proton = Hydrogen
- 2 protons = Helium
- 3 protons = Lithium ... and so on
Number of electrons = Number of protons
All elements are Chemically Distinct
Top Ten Most Abundant Elements
- 10) Sulfur
- 9) Magnesium
- 8) Iron
- 7) Silicon
- 6) Nitrogen
- 5) Neon
- 4) Carbon
- 3) Oxygen
- 2) Helium
- 1) Hydrogen
In general, the ordinary matter in the Universe is mostly Hydrogen and
Helium (roughly 75% and 24%, respectively), and 1% every other element.
Known Elements
There are 117 elements currently known from laboratory studies:
- 87 are metals
- 11 are gasses
- 2 occur as liquids (Bromine & Mercury)
- 26 are radioactive
- 24 are made only in the lab
In addition, each element can have a number of isotopes.
The two newest members of the table of the elements, with atomic numbers
of 113 and 115, were recently made in accelerators in Russia.
Isotopes
A given element can have many different Isotopes
- Same number of protons.
- Different number of neutrons.
Example: Carbon Isotopes:
- 12C has 6 protons and 6 neutrons
- 13C has 6 protons and 7 neutrons
- 14C has 6 protons and 8 neutrons
All isotopes of a given element are chemically identical, but they have
different masses.
Radioactivity
If a nucleus has too many or too few neutrons, it becomes unstable to
radioactive decay
Examples:
3H (1p+2n) -> 3He (2p+1n) + e- + neutrino
14C (6p+8n) -> 14N (7p+7n) + e- + neutrino
(basis of radioactive carbon dating)
Free neutrons are also unstable:
n -> p + e- + neutrino
All of the above are examples of "beta decay", in which one
of the neutrons turns into a proton, shedding its excess mass by
spitting out an electron and a neutrino.
The Fundamental Forces
All interactions among matter are governed by four fundamental forces:
- Strong & Weak Nuclear Forces
- Bind protons to neutrons inside nuclei
- Mediate nuclear reactions and radioactivity
- Electromagnetic Force
- Binds electrons to nuclei inside atoms, and atoms to atoms inside
molecules
- Mediates chemical reactions
- Gravitational Force
- Binds massive objects together on large scales
- Mediates orbital motions
Gravitation
Gravitation binds matter to matter over long distances:
- Long-range attractive force
- Weakest force of nature.
- Obeys the Inverse Square Law of distance:
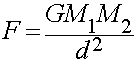
The Force of Gravity between any two objects depends only upon:
The masses of the two objects:
- More massive objects exert a stronger the gravitational force.
The distance between them:
- The force gets stronger as the two objects move closer together.
- The force gets weaker as the two objects move farther apart.
It does not depend on the shapes, colors, or compositions of the
objects.
Kepler's 3rd (or "Harmonic") Law of orbital motions
P2 = a3
only applies to planets orbiting our Sun.
Newton, however, derived a more general version
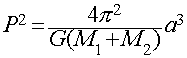
This is a universal form of Kepler's 3rd law applicable to any
two massive objects orbiting each other anywhere in the Universe.
- The important quantities in this form are:
- P = period of the orbit
- a = semi-major axis of the orbit
- M1+M2 = combined masses of the two bodies
This gives us a way of measuring the masses of objects from observation
of their orbital period and semi-major axis.
In the simple case of a tiny mass in a circular orbit around a more
massive body we can simplify the formula above to define the smaller
object's Circular Speed:
- Circular Speed
- Speed needed to sustain a circular orbit at a given distance from
a massive object:
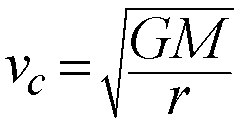
The circular speed depends on two things:
- The mass of the larger parent body (M)
- The radius of the orbit (r)
This gives us another way to measure masses for objects in circular orbits
around larger bodies, now measuring orbital speeds (Doppler Effect) and
orbital radii.
In general, orbital motions are the most important way we have to
estimate the masses of astronomical objects.
Another consequence of gravitating bodies is that there is a minimum
speed an object must be given in order for it to escape from the gravity
of its parent body.
This is called the Escape Speed:
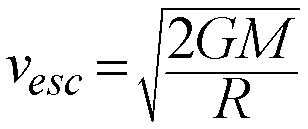
The escape speed depends on the Mass of the body (M) and its radius (R).
Basically, the stronger the gravity at the surface of a body, the faster
you have to be going to escape from its gravitational clutches.
Examples:
- Earth's Surface
- Vesc = 11.2 km/sec (40,320 km/hr)
- Sun's Surface
- Vesc = 615 km/sec (~2.2 Million km/hr)
Imagine you wanted to take apart a gravitating body. You might do it
piece-by-piece, taking a rock and throwing it with the escape speed.
As the body got steadily smaller, the escape speed would be less.
If you added up all the energy you would need for each piece, you would
derive the Gravitational Binding Energy for the body:
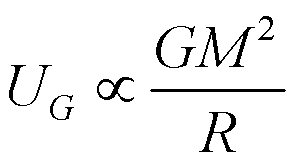
This depends on two quantities: the Mass (M) and the
Radius (R) of the body.
The formula above is a "proportionality", it tells us how the binding
energy scales with the mass and radius of the object. The constant
out front we need depends on details of how matter is distributed in
the object. For example, a sphere of uniform density would have a
constant of 3/5.
For the Earth, the Gravitational Binding Energy is about
2x1032 Joules, or about 12 days of the Sun's total energy
output!
Implications
For objects of the same Radius but with different masses, the more
massive object will have
- Faster orbital and escape speeds
- Greater gravitational binding energy ("more tightly bound")
For objects of the same Mass but different Radii, the larger object
(bigger radius) will have:
- Slower circular and escape speeds
- Less gravitational binding energy ("less tightly bound")
Gravity: The Universal Glue
Gravity is the force that rules in the domain of astrophysics:
- Holds planets and stars together
- Controls the orbits of moons around planets
- Controls the orbits of planets around stars
- Binds stars into galaxies
- Binds galaxies into groups and clusters
- Binds galaxy clusters into superclusters
- Binds the Universe together...
Throughout this course we will be exploring the consequences of
gravitation.
Return to [
Introduction Index
|
Astronomy 162 Main Page
]
Updated: 2006 January 4
Copyright © Richard W. Pogge,
All Rights Reserved.